ISO Management
The Journey
For a number of years we have developed proprietary ISO Quality Management systems for SME’s seeking help managing their ISO accreditation; either as standalone systems, or as a part of larger and more specific business management suites. These systems have taken many forms, but have ultimately shared the same core values. We have hands on experience working on systems in relation to ISO 9001, ISO 13485 and most recently ISO 26000.
The Solution
Having worked on such a wide breadth of systems, we have identified the key aspects required in a large number of accreditations, and created a skeleton ISO system that we have used as the core building blocks for custom builds.
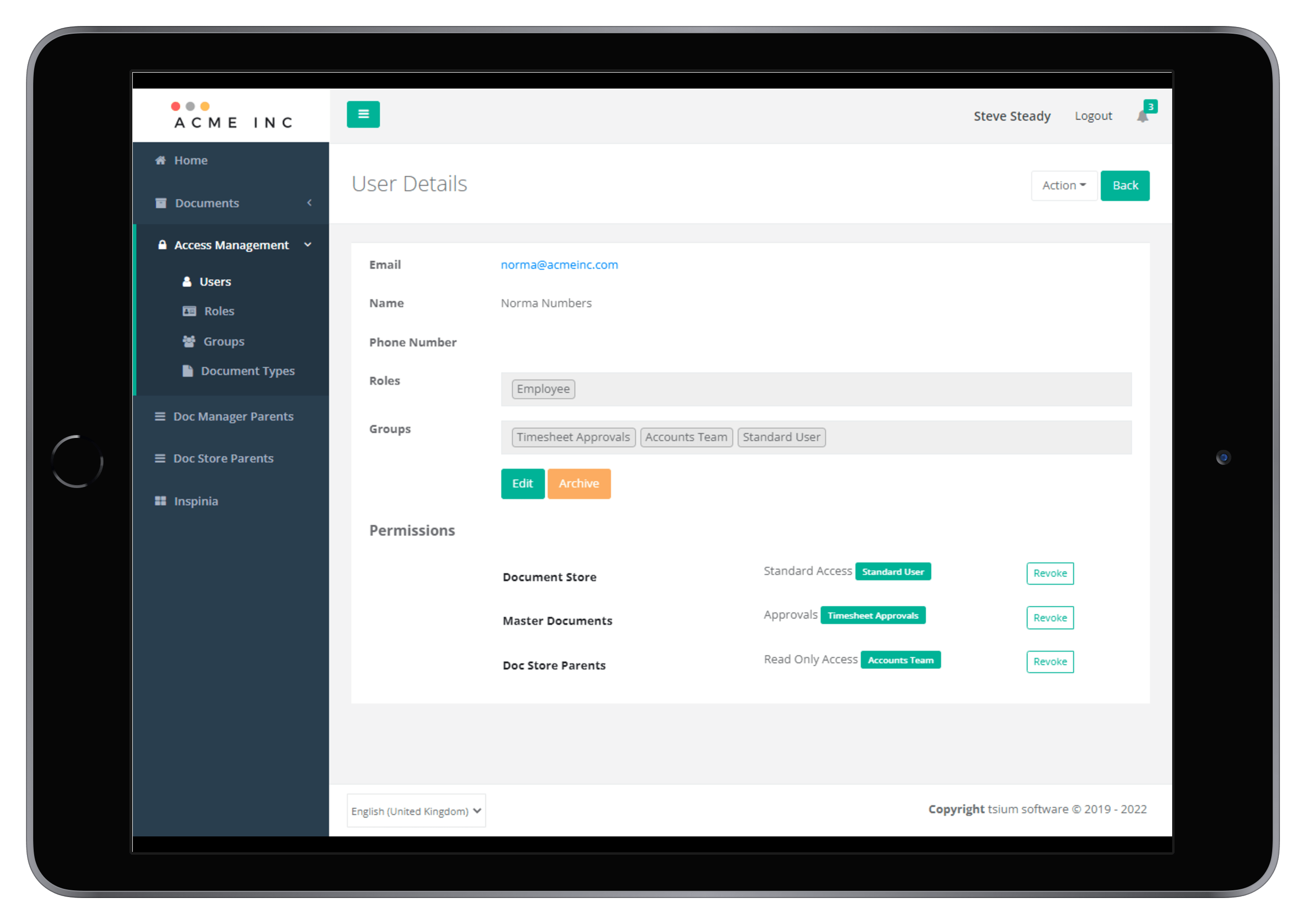
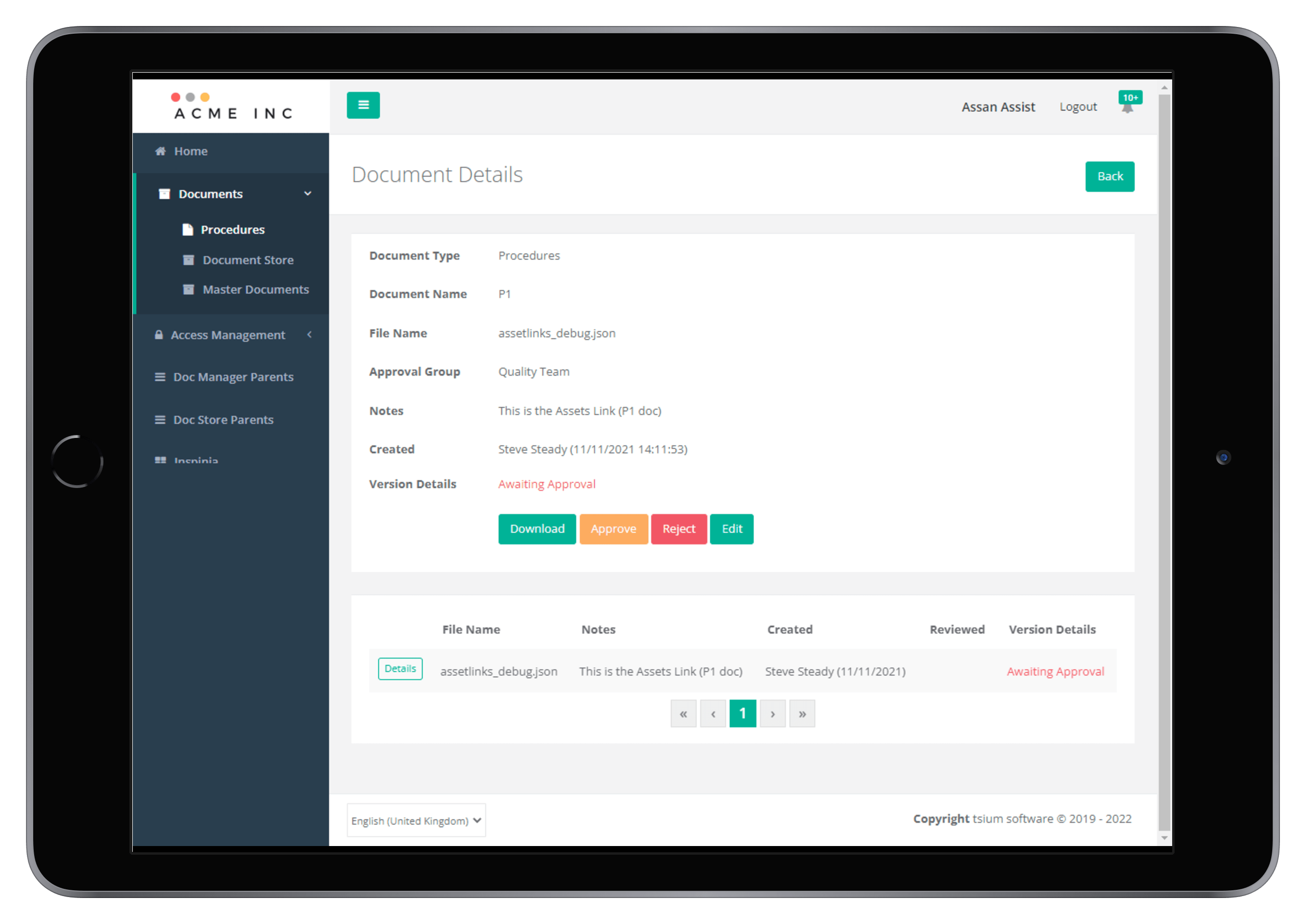
It includes a strong and flexible access management framework, version controlled document management, a document store repository, and configurable workflow task management.
How We Can Help
ISO 9001 (QMS)
Quality Management Systems
- Document Management
- Quality Policy
- Procedures
- Audit Schedule and Reporting
- Management Review
- Resource Management
- Training Log
- Approved Suppliers
- Asset Register
- Workflows
- Customer Satisfaction/Complaints
- Non-Conformance Reports (NCR-CAPA)
- Calibration Records and Schedules
ISO 13485 (MDD)
Medical Device Directive
- Quality Manual
- Document Control
- Work Instructions
- Drawing Register
- Quality Procedures
- Supplier Assessments
- Batch Manufacturing Records
- Complaint logging
- Risk Assessments
ISO 14001 & 26000 (ESG)
Environmental, Social and Governance
- Social responsibility
- Stakeholder reporting
- Human rights
- Labour practices
- Environmental impact
- Fair operating practices
- Consumer issues
- Community involvement & development
- Supply chain